Life history timing and the origin and maintenance of biodiversity
Phenology, or seasonal timing, is a critical component of adaptation for organisms in temperate environments. From subtropical forests to the arctic tundra, animals, plants, and microbes are challenged by shifting conditions throughout the year. Abiotic conditions such as temperature and precipitation along with seasonal changes in biotic factors, such as the presence or abundance of prey, mutualists, competitors, or parasites may restrict an organism’s ability to thrive throughout the year. We expect natural selection to act strongly on traits that allow organisms to mitigate these seasonal challenges, by allowing them to weather unfavorable conditions and synchronize key life stages – like growth and reproduction, with the right temporal windows.
The evolution of seasonal timing is an important facet of my lab’s dual central questions of ‘how does new biodiversity come about?’ and ‘how is biological variation maintained in the face of environmental change?’. For the first part, because reproduction is often highly seasonal, phenological adaptation may be a potent driver of speciation. If adaptation to a new ecological opportunity involves a shift in life cycle timing, the resulting offset in mating seasons can rapidly drive reproductive isolation – placing populations on separate evolutionary trajectories on the path to become separate species. Seasonality may therefore be a critical axis of diversification for some groups of organisms, with diversity in seasonal niches setting the stage for temporal adaptive radiations.
For the second question, altered phenology is expected to be one of (if not the) most important ecological ramifications of climate change. For many organisms, particularly ectotherms like insects, the direct effects of increased temperatures in the coming decades may not pose existential threats to populations, in terms of being able to survive and grow in slightly warmer conditions. However, the changing climate may have drastic effects on the environmental cues that organisms use to maintain adaptive life cycle timing. An increase in a few degrees across seasons, shortened winters, or increased variability during seasonal transitions may pull the phenological rug out from under ecological communities, driving maladaptive life history responses or decoupling seasonal synchrony across closely interacting organisms.
Much of the work in my lab focuses on understanding the ecological and genomic factors that promote or constrain rapid adaptation to novel seasonal conditions. We are interested in questions such as the eco-physiological basis of variation in life history timing, how standing genetic variation underlying seasonal adaptation is maintained in populations, the genomic architecture of dormancy traits, the role of phenological divergence in adaptive radiations, and predicting how populations and communities will respond to future seasonal regimes. These questions of seasonal adaptation are relevant to all of the study systems our groups works with, including seed dispersing ants and gall-former/parasitoid communities (see below), but this forms the basis of our primary work with Rhagoletis flies: a model system for both ecological speciation-in-action and rapid phenological adaptation.
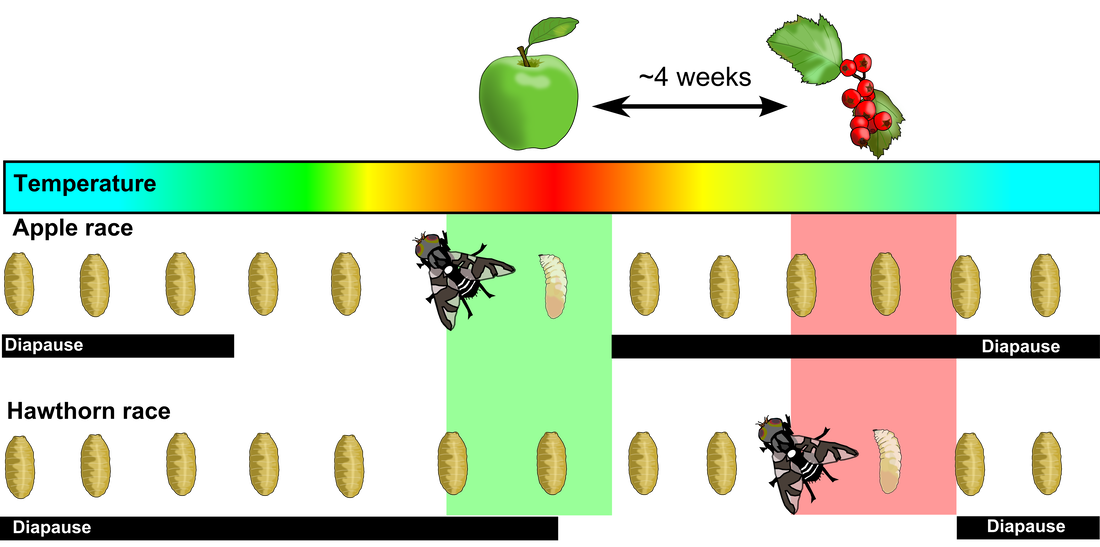
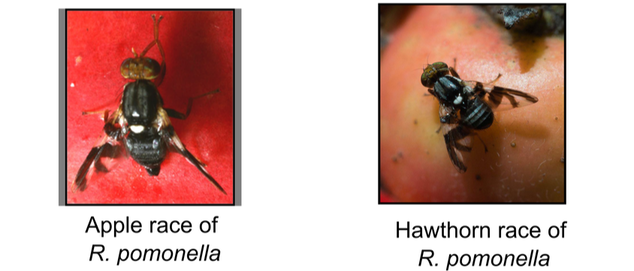
Rhagoletis pomonella is a specialist Tephritid fruit fly that ancestrally infested the fruit of hawthorn trees in eastern North America. In the 1850s, a population of these flies shifted to specializing on infesting apples - which had been introduced by European colonizers two centuries earlier. Shifting to this novel host required adaptation in two key traits: 1) chemosensory behavioral adaptation shaping attraction and avoidance to the fruit surface volatiles used by the flies to find mating and oviposition sites and 2) dormancy-mediated life history timing, synchronizing the short-lived adult life stage with the brief period of available ripe host fruit, which occurs 3-4 weeks earlier for apple compared to hawthorn. Both ecological traits play a direct role in the flies' system of mating, driving both prezygotic and postzygotic reproductive isolation. Gene flow between apple and hawthorn flies in places where the trees grow side-by-side is limited to ~4% per generation, placing these flies in an intermdiate phase of the speciaiton process in just the past ~160 years.
This evolutionary shift to an earlier life cycle timing during recent historical times is closely analagous to what we expect many populations may be faced with in the coming decades under climate change. Our work includes both further elucidating the classic speciation story in Rhagoletis and also developing this as a powerful system for studying rapid adaptation to climate change. Current projects include: testing for phenotypic and genomic responses to simulated climate change using environmental chambers that mimic natural soil temperature conditions for R. pomonella pupae, determining how genetic variation underlying seasonal adaptation is maintained within and across populations, investigating how different facets of climate change - such as shortened winters and increased volatility in winter/spring transitions may disrupt adaptive life history timing, and integrating phenotypic and population genomic data at broad geographic scales to predict thresholds of vulnerability to altered seasonal regimes across populations.